Do you sometimes find yourself faced with soaps that don't lather? Do you like mousse? But have you ever been interested in the science behind this foam? And why is it useful for shaving?
How to create foam?
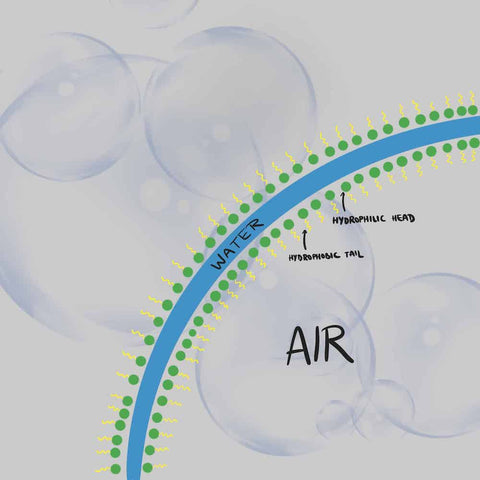
As children, we had fun blowing soap bubbles; All you had to do was mix dishwashing liquid with water, dip a circle, and blow. But how do these bubbles work and what are they made of?
What about the foam then?
From a scientific point of view, foam... It's gas bubbles separated by a liquid! The foam created by soap in our daily lives is therefore air bubbles separated from each other by a liquid, often water. Foam is millions of air bubbles attached to each other by a continuous film formed of water trapped between two layers of soap molecules.
And shaving in all this?
Soap foam has a very important role to play in classic shaving, thanks to its water content. Since foam is water between thin layers of soap, the more foam, the more water. The denser the foam created by the soap, the more water there will be (constituting the continuous film), and the less air there will be. Water is essential in a quality shave, because it softens the hair which becomes soaked with water, and it prevents dehydration of the skin.


